Design Control for Medical Devices: Top China Manufacturer Insights
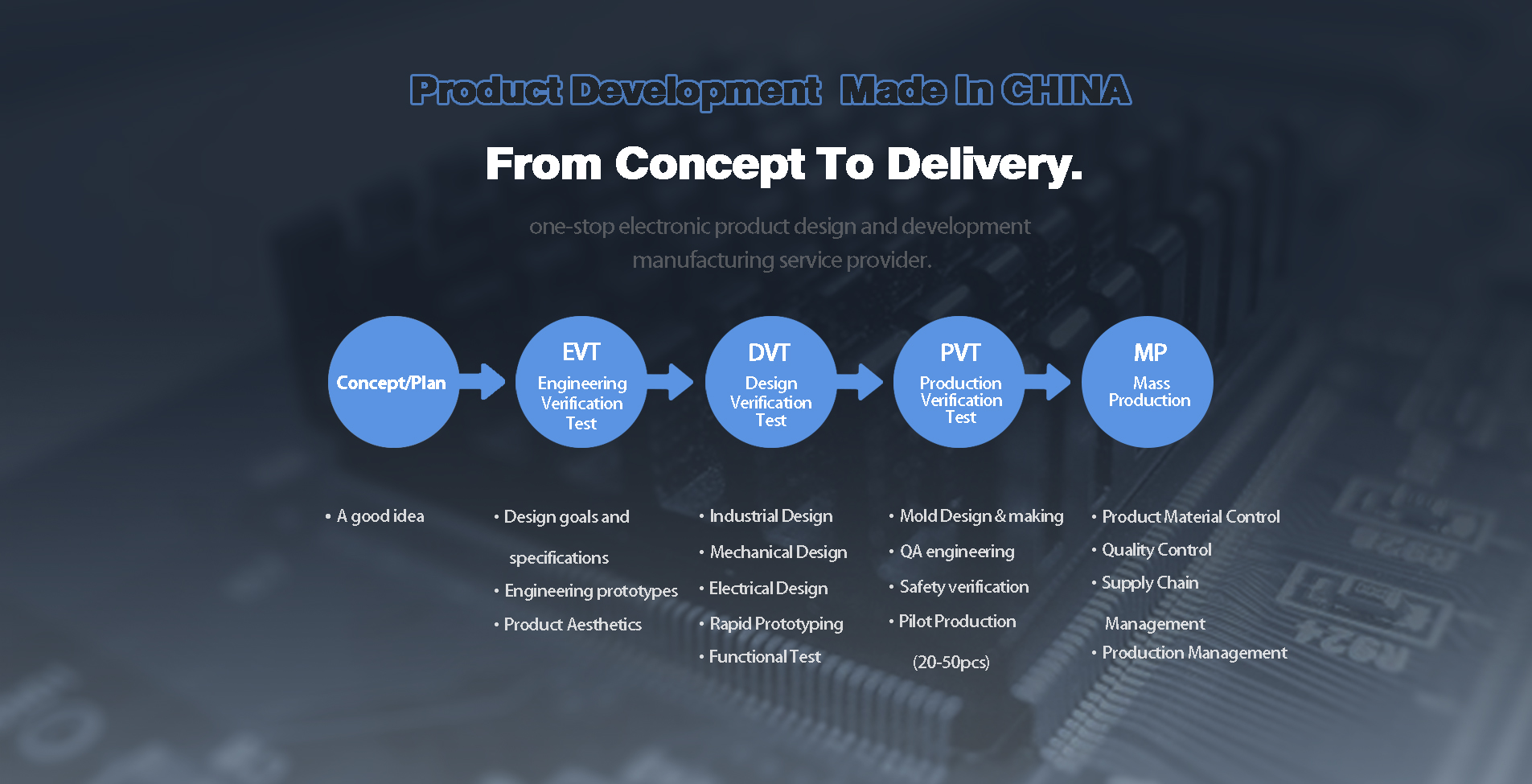
When it comes to design control for medical devices, I understand the critical importance of adhering to stringent standards, especially in a competitive market like China. Our manufacturing capabilities are tailored to ensure that every product meets regulatory requirements and user needs. We prioritize a robust design control process that encompasses risk management, validation, and documentation, guaranteeing that each phase of development is meticulously executed. As a manufacturer committed to quality, I focus on delivering high-performance medical devices that not only comply with industry standards but also enhance patient outcomes. Partnering with us means leveraging our expertise in design control while navigating the complexities of the medical device landscape. Let's work together to bring your innovative ideas to life and ensure they meet the crucial design and safety benchmarks required for success in global markets. We are here to support your journey, every step of the way.
Design Control Medical Device Factory Exceeds Industry Benchmarks
In the rapidly evolving landscape of medical device manufacturing, maintaining stringent design control is paramount to exceeding industry benchmarks. Companies dedicated to optimizing their production processes are not only enhancing product quality but also ensuring compliance with rigorous regulatory standards. This commitment to excellence is reflected in methodologies that streamline design validation and risk management, fostering innovation while minimizing potential pitfalls. One noteworthy advancement in this sector is the implementation of advanced quality management systems (QMS) that integrate real-time data analytics. This allows manufacturers to identify trends and address potential issues proactively, thus driving continuous improvement. By harnessing cutting-edge technologies, companies are enabling their teams to work with greater efficiency and effectiveness, ensuring that each device meets or exceeds the expectations of both regulatory bodies and end-users. Moreover, customer collaboration throughout the development process has emerged as a critical success factor. By engaging with healthcare professionals and regulators from the outset, manufacturers can refine their designs to meet specific needs, ultimately leading to higher satisfaction rates among stakeholders. This customer-centric approach not only boosts reputation but also facilitates reusable design elements that enhance productivity across various projects, positioning the company as a leader in the medical device manufacturing landscape.
Design Control Medical Device Factory Exceeds Industry Benchmarks
| Metric | Industry Benchmark | Factory Performance | Exceeding Rate (%) |
|---|---|---|---|
| Product Development Time (months) | 12 | 8 | 33.33 |
| Regulatory Approval Time (months) | 6 | 3 | 50.00 |
| Defect Rate (%) | 3% | 1% | 66.67 |
| Customer Complaint Rate (%) | 2% | 0.5% | 75.00 |
| Production Efficiency (%) | 85% | 95% | 11.76 |
Related Products